|
|
|
_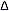 J
= 0, J
= 0, 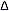 J
= ±2 J
= ±2 |
(8) |
_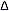 J
= 0 transitions correspond to the intense Rayleigh scattering. J
= 0 transitions correspond to the intense Rayleigh scattering.
Thus if the molecule gains rotational energy from the photon
during collision, a series of S branch lines is apparent
to low wavenumber side of the exciting line which are called
Stokes lines. An S-branch can also be formed
on the Anti-Stokes side of the excitation line.
Q If Stokes transitions form an S branch, how can Anti-Stokes
transitions be labelled S as well? |
|