|
|
|
Quantum
Theory of Raman effect
As a stream of photons collides with a particular molecule the photons will
be deflected without change in energy if collisions are perfectly elastic. If energy
is exchanged between photon and molecule,
the collision is said to be inelastic. The molecule can gain or lose
discrete amounts of energy in accordance with quantal laws - the energy
must coincide with a transition between two molecular energy levels.
The Classical Raman Effect
The distortion of a molecule in an electric field is determined by
its polarisability 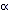 .
If the strength of the field is .
If the strength of the field is 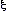 ,
in addition to any dipole moment it may already have the molecule
acquires an additional dipole moment: ,
in addition to any dipole moment it may already have the molecule
acquires an additional dipole moment:
|